In September I had the pleasure to be one of a handful of researchers invited to participate in the National Heart, Lung, and Blood Institute’s working group on early translational research needs in blood science (described in my previous post: Part 1). Below are the working group’s recommendations:
To address the four stated needs, the institute has developed a framework with four goals in mind:
- Determine the feasibility of translational research projects
- Advance transformative products to human trials
- Advance transformative technology platforms
- Overcome disease-specific translational research barriers
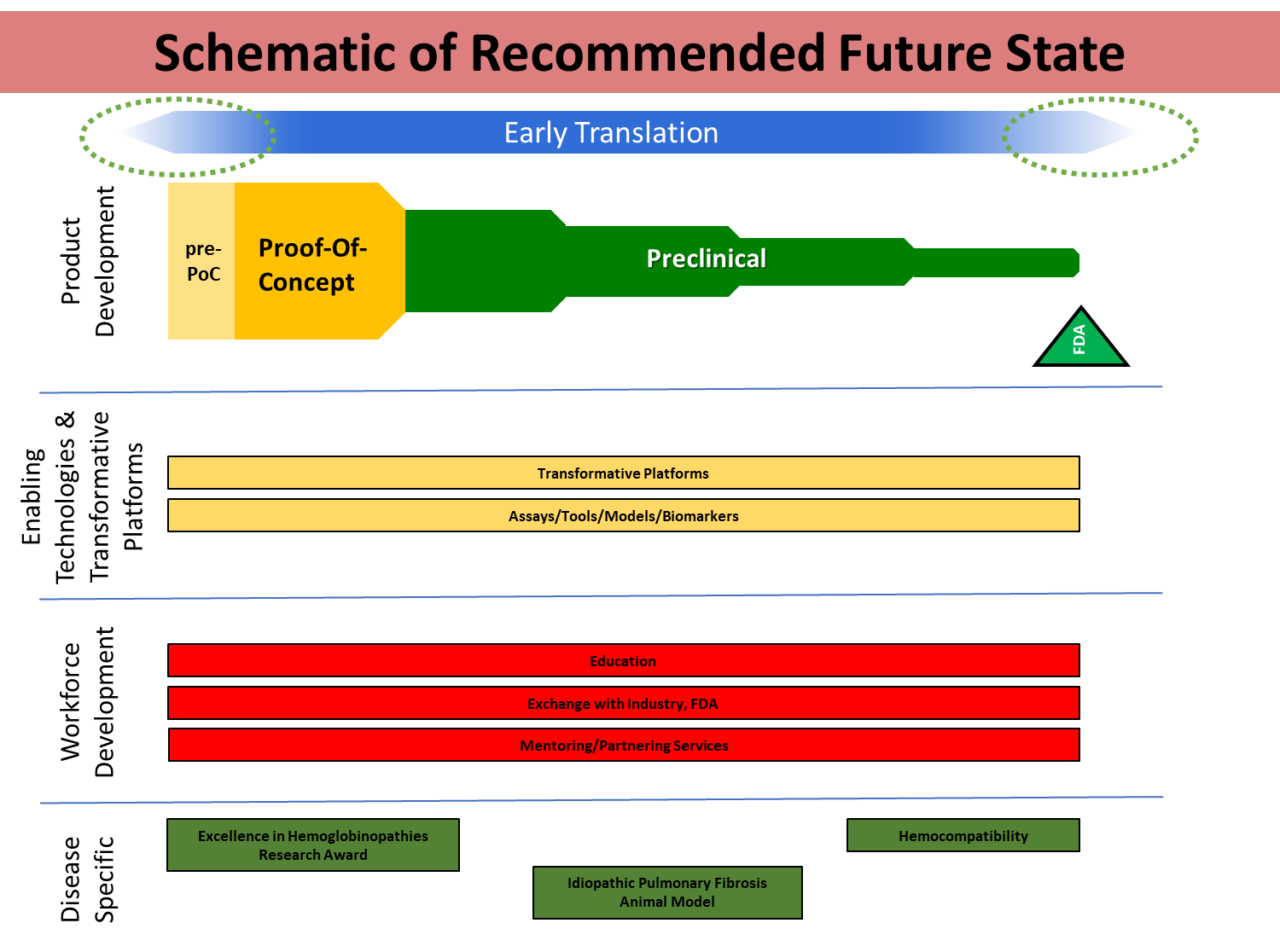
With this background in mind, the working group asked us six specific questions:
- What have been the biggest gaps in your translational research endeavor?
- Funding?
- Lack of core facilities at your institution?
- Lack of consultative expertise, e.g. medicinal chemistry, toxicology, etc?
- What do you see as essential elements for proof-of-concept and pre-proof-of-concept research that are non-hypothesis driven and outside the scope of R01 grants? Do you see the need for other funding mechanisms such as the R21 Pilot/Exploratory grant?
- What gaps did NHLBI-funded translational research programs such as VITA, TACTIC, EHRA, and TRC-THD fill that were not present or available to you through your collaborators or your institution?
- What important translational research services have you been unable to build/develop at your institution?
- What do you see as blood-specific translational research needs (e.g. based on the unique nature of red blood cells, platelets, etc)?
- What do you see as blood disease-specific translational research needs (e.g. sickle cell disease, thrombosis, TTP, etc)?
It was refreshing to note that without prior coordination, every invited participant presented the same overarching conclusions – a marked departure from my usual experience than any three professors asked a yes/no question will give you four discrepant opinions. More refreshing still was the feeling that the NHLBI was actually listening.
The highlights include:
- Need for continued funding, if the group is hitting all of its milestones through the technology life-cycle, (a mechanism for competitive ongoing support) to avoid losing established expertise/groups on promising research programs.
This recommendation was born out of the observation that discontinuity in funding leads to losing trained personnel and trainees through dismantling of collaborations. Although not generally practical to conduct through conventional grant committees since no standing study section could evaluate or assess project significance for certain types of translational projects, the decisions on which programs should be competitively renewed, dropped or added could be accomplished through the use of continuing competitive reviews by ad hoc panels with program oversight by NHLBI Council.
- Need for expedited pilot/exploratory grant awards without standard deadlines.
- Need to require organization of projects around milestone-based deliverables (go/no-go points) that can be used to direct continuation of project funding.
- While establishment of additional core facilities was not identified as an area of need, support/contribution to charge-backs for the most expensive elements of core facilities (eg, clinical grade cell banking, animal care, next-gen sequencing, advanced imaging, flow-cytometry/Ab purchase) were considered crucial.
- Biannual one-day meetings to bring collaborative groups together to share progress and direct next phases of development.
- Need to establish a coordinating central website to link resources across different divisions/institutions/program that must be easy to navigate and kept up-to-date.
My next post on this topic will describe our further recommendations to the working group, including the role Dave and I believe the federal funding agencies should play in the future translational research in the sciences.
Our federal funding agencies read these posts. Now is an excellent time to share your thoughts.
